Ansys Paves the Way to Personalized Medicine
Aliyah Mallak, Senior Marketing Communications Writer, Ansys
 When we need an orthopedic implant or a cardiovascular device such as a stent, we may assume it’s personalized, customized, or selected for us. Yet almost all orthopedic and cardiovascular surgeons use off-the shelf products and select them based on experience.
When we need an orthopedic implant or a cardiovascular device such as a stent, we may assume it’s personalized, customized, or selected for us. Yet almost all orthopedic and cardiovascular surgeons use off-the shelf products and select them based on experience.
They are rarely, if ever, personalized for an individual patient. The one-size-fits-all approach to healthcare fails to recognize the significant differences among the anatomical and physiological characteristics of individual patients. This creates inefficiencies and cost overruns, but also affects the quality of care provided. Personalized healthcare could help provide optimal, patient-specific treatment plans, from diagnosis to therapy.
While this concept might seem like science fiction, advanced technology is poised to speed up patient recovery and improve the quality of life for people around the world. Smart medical devices and wearables are already bridging the gap between hardware and software, with heart rate and blood pressure monitoring, sleep cycle tracking, and more.
Yet personalized healthcare still requires a significant paradigm shift and new technology tool kits for collecting and creating data used to customize treatment. Biomechanical simulations offer a fast and straightforward solution for personalization at the point of care to treat patients with devices or implants that interact optimally with their body. In this way, healthcare providers can create truly personalized treatment plans and rule out possible complications.
One company leading the charge to personalized medicine is Simq — a corporate spinoff from Ansys Channel Partner CADFEM International. Simq develops easy-to-use healthcare applications to accelerate the adoption of personalized medicine. By combining biomechanics and in silico technologies, Simq provides a compliant development infrastructure to democratize physics-based simulation and give the healthcare community access to more information and personalized insights.
Their team of experts is committed to delivering the highest quality solutions to make the potential of digital twin simulation — a patient-specific computer model — artificial intelligence, and machine learning broadly available.
DEMOCRATIZING SIMULATION FOR MEDICAL PROFESSIONALS
While simulation is a tremendous asset in many industries, companies may be reluctant to hire experienced engineers to perform simulations. With high costs and long 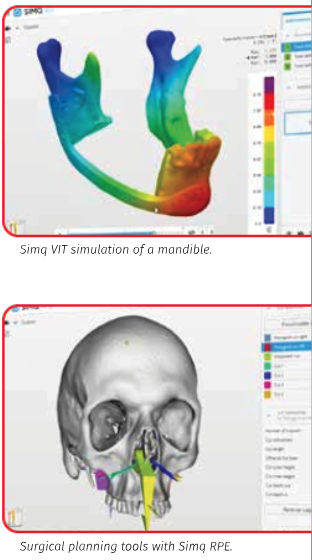 delays prominent in the healthcare industry, most companies don’t have the budget to hire engineers, nor the time to train healthcare professionals to use simulation software.
delays prominent in the healthcare industry, most companies don’t have the budget to hire engineers, nor the time to train healthcare professionals to use simulation software.
As a company, Simq offers several solutions for specific purposes, such as virtual implant testing (Simq VIT), surgical planning tools for complex osteosynthesis (Simq OSP) and rapid palatal expansion (Simq RPE), and a medical device to improve diagnosis in sleep apnea patients (Simq OSA). They use PyAnsys — a technology combining Python and Ansys solutions to democratize simulation — to assist biomedical engineers, and eventually medical professionals, with diagnostics, research and development, treatment planning, and implant testing.
By giving Simq access to Ansys’ physics-based engineering tools, Simq developers can focus on the implementation and adoption of digital twins in medicine and regulatory requirements. Simq is developing applications with an easy-to-use, easy-to interpret user interface (UI), which prevents the need to hire specialty engineers in hospitals or train medical professionals on all the intricacies of simulation.
“PyAnsys is a game-changer for the development of Simq products and our digital twin technologies,” says Alexander Volf, Chief Technology Officer of Simq. “PyAnsys provides a modern, state-of-the-art Pythonic interface. This is a huge advantage for developers. Beyond its immediate usability, it also opens access to the extensive capabilities of the Python ecosystem in the simulation process, as well as the combination of simulation with mathematical, statistical and machine learning methods.”
SIMULATION EMPLOYED AS A MEDICAL DEVICE
Meeting regulatory requirements is one challenge faced by Simq and its customers. Medical devices of any kind are subject to approval from regulatory authorities such as the Food and Drug Administration (FDA) in the United States or the notified bodies in Europe.
To approve a medical device, the manufacturer must provide extensive documentation and clinical data showing that the device is safe and effective. But Simq doesn’t produce physical devices; their customers do. So, are they responsible for following regulatory guidelines? Yes, they are. As with medical devices, whether software qualifies as a medical device is determined by the manufacturer’s intended purpose for the software. From a regulatory perspective, medical software is subject to the same requirements as a physical medical device.
Accordingly, medical software must meet certain standards. Simq’s VIT, OSP and RPE medical software solutions enable medical device manufacturers to check their devices for compliance and work towards personalized medicine. They provide verification, validation and uncertainty quantification following FDA standards for patient-specific simulation models and quality assurance for products according to ISO 13485. Simq can reuse their regulatory framework and validation process for precision medicine and digital twins, which helps to speed up the development processes.
To ensure that as many patients as possible benefit from Simq’s solutions, they make the basic technologies available to other medical device manufacturers. Instead of starting from scratch every time, manufacturers can use Simq’s development framework, which saves time and money through reusable technical documentation. This gives customers valuable resources so they can focus on the next big innovative medical device.